Ligand Binding to PXR

 |
|
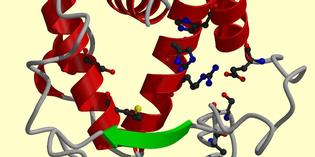
The binding pocket of hPXR. |
Overview
 The
human pregnane X receptor (hPXR) is a nuclear receptor that binds to various
ligands, regulating the breakdown of drugs in the human body. To study drug-drug
interactions, we are investigating a method that predicts potential
ligand binding conformations in the binding pocket of hPXR. The
human pregnane X receptor (hPXR) is a nuclear receptor that binds to various
ligands, regulating the breakdown of drugs in the human body. To study drug-drug
interactions, we are investigating a method that predicts potential
ligand binding conformations in the binding pocket of hPXR.

Binding Conformations from
the Geometry of Hydrogen Bonds
 The
binding pocket of hPXR contains eight polar residues which are key in binding
ligands. These polar atoms can potentially share hydrogens with the polar atoms of
ligands, forming hydrogen bonds. The
binding pocket of hPXR contains eight polar residues which are key in binding
ligands. These polar atoms can potentially share hydrogens with the polar atoms of
ligands, forming hydrogen bonds.
 |
 |
 |
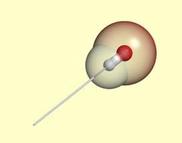
| A stick for the
preferred bond direction from a donor polar atom. |
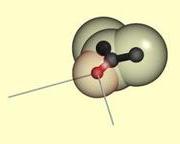
Two sticks for the
preferred bond directions from an sp2 hybridized acceptor polar atom. |
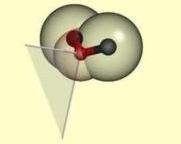
A wedge for the
preferred bond directions from an sp3 hybridized acceptor polar atom. |
Hydrogen bonds have a preferred geometry
that depends on the chemical neighborhood of the polar atoms. We model the observed
preferred geometry for hydrogen bonding by sticks and wedges. A hydrogen bond is
made if two polar atoms are close, and a donor stick is aligned with an acceptor
stick or wedge. These geometric constraints are formulated in an
optimization problem, whose solutions are ligand conformations that establish at least two
hydrogen bonds between a ligand and the binding pocket. We then check the candidate
conformations for steric hindrance using a hierarchical collision-detection algorithm and retain the valid conformations
in the
binding pocket.
 |
 |
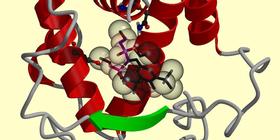
| A conformation
generated of SR12813, generated by our program. It establishes
interactions similar to those observed experimentally. |
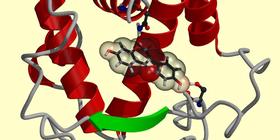
A
conformation generated of coumestrol, generated by our program. |
References

People
Acknowledgement
This work has been partially funded by an
NSF-ITR grant.
 |

![]() The
human pregnane X receptor (hPXR) is a nuclear receptor that binds to various
ligands, regulating the breakdown of drugs in the human body. To study drug-drug
interactions, we are investigating a method that predicts potential
ligand binding conformations in the binding pocket of hPXR.
The
human pregnane X receptor (hPXR) is a nuclear receptor that binds to various
ligands, regulating the breakdown of drugs in the human body. To study drug-drug
interactions, we are investigating a method that predicts potential
ligand binding conformations in the binding pocket of hPXR.
![]()
![]() The
binding pocket of hPXR contains eight polar residues which are key in binding
ligands. These polar atoms can potentially share hydrogens with the polar atoms of
ligands, forming hydrogen bonds.
The
binding pocket of hPXR contains eight polar residues which are key in binding
ligands. These polar atoms can potentially share hydrogens with the polar atoms of
ligands, forming hydrogen bonds.





![]()
![]()